

7.2 and 7.3 gives data tables and graphs for the s-block groups and 7.4 discusses the general group trends in. All alkali metals are highly reactive with water, halogens and acids.Īll the alkaline earth metals found are present in nature. Group I Group II Part 7.1 gives an introduction to the chemistry of Group 1 Alkali Metals and Group 2 Alkaline Earth Metals via their outer electron configuration. Beryllium is sufficiently hard to scratch glass, but barium is only slightly harder than lead. In the light of the above statements, choose the most appropriate answer from the options given below. o Lithium sodium and potassium metal are produced by electrolysis of the molten chlorides. Only mercury (38.9 ☌, or 38.02 ☏) has a lower melting point than caesium, and the low melting points of alkali metals are due to their vast interatomic distances and weak. All alkaline earth metal atoms have two electrons in their valence shell preceded by the noble gas configuration.
#Alkaline earth metal reactivity trend free
Hence, it does not occur in free state however, is widely distributed within the nature in combined state as silicates, carbonates, sulphates, and phosphates. Statement II : Solubility of alkaline earth metal hydroxides in water increases down the group. Each metal reacts with dilute hydrochloric acid, producing bubbles of hydrogen gas and a colorless solution of the metal chloride: X + 2HCl XCl2 +H2 X + 2 H C l X C l 2 + H 2. The alkali metals have the lowest melting points of any nongaseous group in the periodic table, ranging from 179☌ (354☏) for lithium to 28.5☌ (83.3☏) for caesium. Alkaline earth metals are highly reactive metals. They have a gray-white lustre when freshly cut but tarnish readily in air, particularly the heavier members of the group. Statement I : None of the alkaline earth metal hydroxides dissolve in alkali.
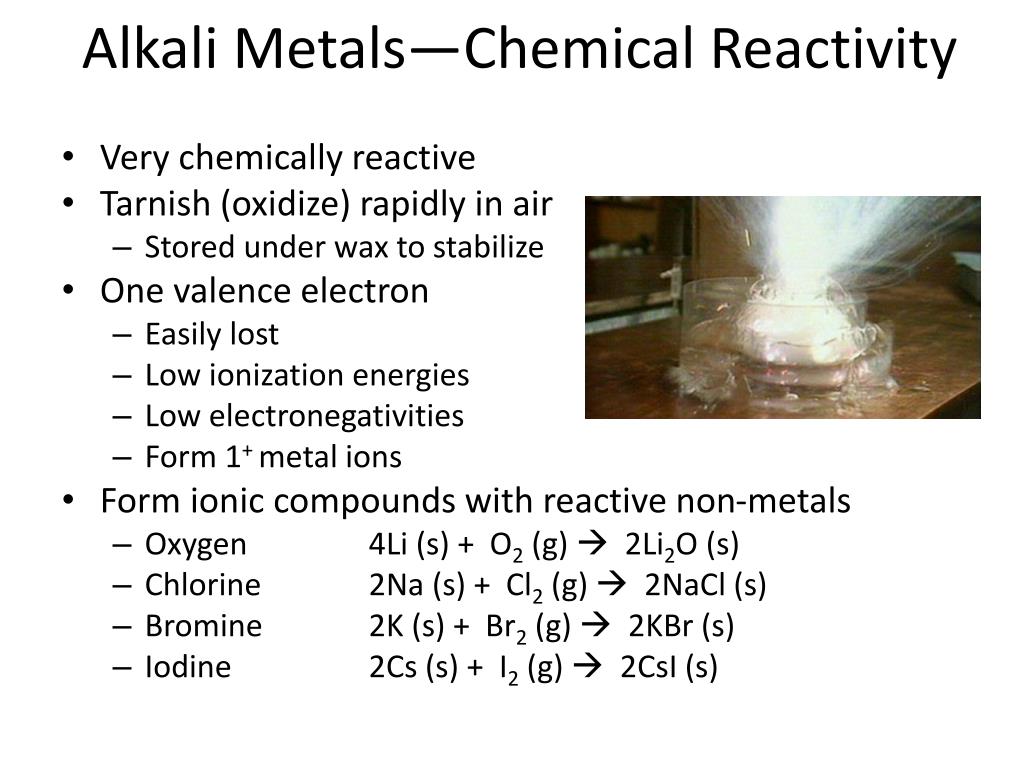
Readily lose their outermost electron to form cations with a charge of +1ĭue to their softness, they can all be easily cut with plastic knives, and their smooth surfaces will quickly lose their gloss in the air due to oxidation. Alkaline-earth metal - Properties, Reactivity, Uses: The alkaline-earth elements are highly metallic and are good conductors of electricity. Highly reactive at standard temperature and pressure Then we have the 7 horizontal rows that go across the periodic table. Some groups include alkali metals (group 1), alkaline earth metals (group 2), halogens (group 17), and noble gases (group 18).
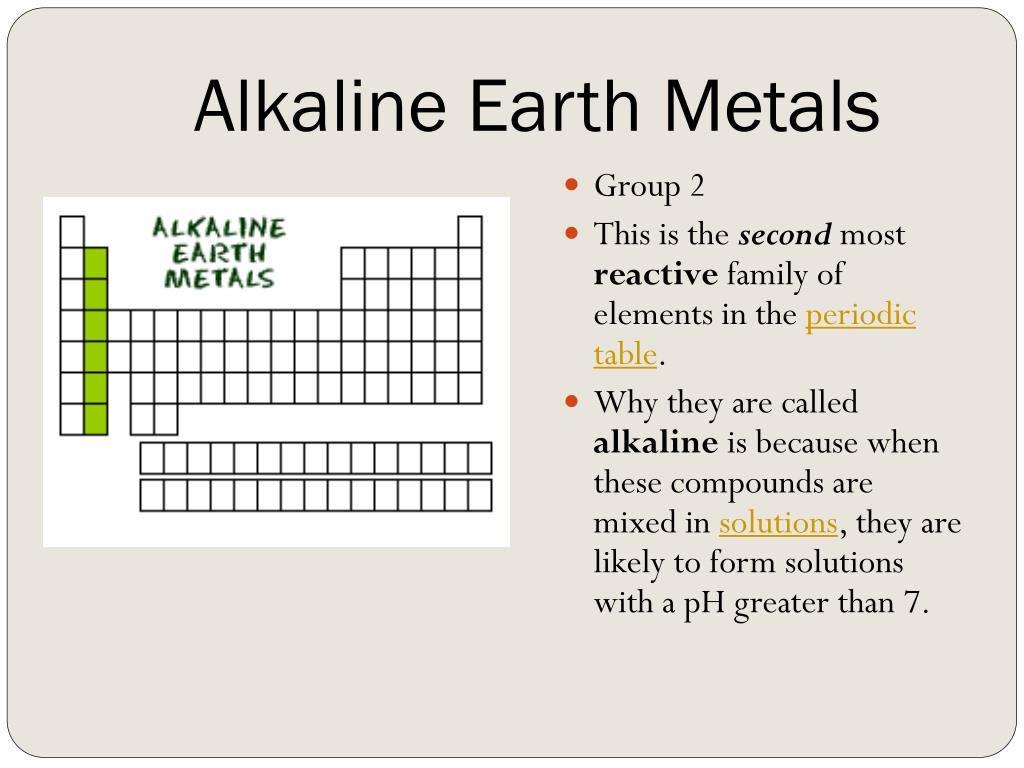
The vertical columns on the periodic table that are numbered 1-18 (from left to right) are called groups or families. (a) Occurrence: Alkaline earth metals are reactive elements and hence do not occur free in nature.


 0 kommentar(er)
0 kommentar(er)
